Case Studies
Case Studies
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 1)
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 2)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 1)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 1)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 3)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 1)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 2)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 3)
.jpg)
To meet the needs of cementing construction in complex well conditions such as deep wells, ultra deep wells, and geothermal wells, a comprehensive molecular structure design concept and functional monomer selection method were adopted. Acrylamide (AM), 2-acrylamide-2-methylpropanesulfonic acid (AMPS), maleic anhydride (MAH), and dimethyldiallylammonium chloride (DMDAAC) were used as polymerization monomers to synthesize a high-temperature resistant zwitterionic polymer type oil well cement retarder HFB-2 through aqueous solution polymerization reaction, with a crude product yield of about 82.7%. The effects of retarder HFB-2 on the thickening time, rheological properties and compressive strength of cement slurry were studied by infrared spectroscopy, gel chromatography and thermogravimetry. The retardation mechanism of retarder HFB-2 was studied by combining phase analysis and microstructure analysis of cement slurry hydration products. The research results show that the retarder HFB-2 has good high temperature retarding characteristics. When the dosage of retarder HFB-2 is 1.2% (mass fraction - the same below) at 220℃, the thickening time of cement slurry is 312 min, and the 24h compressive strength is greater than 14.0 MPa. The formation of calcium silicate hydrate gel and Ca (OH) 2 crystal is mainly inhibited by adsorption and complexation, thus realizing effective control of the hydration rate of oil well cement.
With the ease of oil and gas recovery and the gradual reduction of recoverable oil and gas reserves, oilfield exploration and development have developed towards deep wells, ultra deep wells, and complex wells. However, the complexity of geological conditions has brought many difficulties and challenges to cementing engineering. In the process of cementing operations, in order to ensure smooth construction and meet the requirements of safe pumping, it is often necessary to add retarders to the cementing system to adjust the thickening time of the cement slurry. Since the 1950s, the design and research and development of retarders have gradually attracted widespread attention from researchers and technical construction personnel, and there are various types and relatively mature retarder series available for sale. But with the increasing number of complex well conditions such as deep and ultra deep wells, higher requirements have been put forward for the use of retarders.
As an indispensable functional additive for deep and ultra deep well cementing, retarders have a wide variety of types, mainly including lignin sulfonates, hydroxycarboxylic acids and their salts, sugar compounds, polyols and their derivatives, and inorganic salts. In recent years, 2-acrylamido-2-methylpropanesulfonic acid (AMPS) polymer type retarders have been highly favored. Lei Ting prepared a high-temperature retarder RT300L using AMPS, AM, itaconic acid (IA), and acrylic acid (AA) as polymerization monomers and aqueous solution polymerization method, with a temperature resistance of up to 180℃. Wang Hongke et al. prepared an AMPS type retarder R55L using AMPS, AA, itaconic acid (IA), and N, N-dimethylacrylamide (NNDAM) as polymerization monomers. The prepared cement slurry has the advantages of adjustable thickening time, stable slurry, and low dosage sensitivity within the temperature range of 180℃. Li Junxing et al. prepared oil well cement retarder PAINAS using AMPS, AA, IA, sodium styrene sulfonate (SSS), and N-vinylpyrrolidone (NVP) as raw materials, with a suitable temperature range of 130-160℃. However, it is not difficult to find that the upper temperature resistance limit of the AMPS type oil well cement retarder mentioned above is 180℃, or even lower. The main reason for this may be the decrease in the adsorption capacity of polymer retarders on the surface of oil well cement particles at high temperatures. Therefore, introducing cationic monomers into the molecular structure units of polymer retarders and forming a double helix structure through electrostatic attraction is more conducive to the adsorption of retarders on the surface of cement particles. Peng Zhigang et al. synthesized an organic-inorganic composite high-temperature retarder (HTR-5) using AMPS, AA, and diallyl dimethylammonium chloride (DMDAAC) as polymerization monomers and montmorillonite as active polymerization filler. HTR-5 was used to prepare cement slurry with good retardancy and salt resistance in the temperature range of 150-180℃.
This article is based on the current situation of the use of retarders in the construction process of deep wells, ultra deep wells, and complex wells. Under the guidance of molecular design ideas and free radical polymerization theory, the cationic monomer DMDAAC is introduced through functional monomer optimization and synthesis process optimization. High temperature resistant zwitterionic polymer type oil well cement retarder HFB-2 is prepared by aqueous solution polymerization method. Then, its retarding mechanism is preliminarily explored through microscopic characterization and analysis methods such as scanning electron microscopy and X-ray diffraction analysis.
1. Experimental Section
1.1 Instruments and Reagents
NICOLET5700 infrared spectrometer; IC761 ion chromatograph; STA449F3 Jupiter synchronous thermal analyzer; X Pert PRO MPD X-ray diffractometer; ZEISS EVO MA15 scanning electron microscope.
Acrylamide (AM), 2-acrylamide-2-methylpropanesulfonic acid (AMPS), maleic anhydride (MAH), dimethyldiallylammonium chloride (DMDAAC), sodium bisulfite, sodium hydroxide, anhydrous ethanol, etc. are all analytical pure and purchased from Chengdu Kelong Chemical Reagent Factory; Jiahua G-grade oil well cement, dispersant SJJ-1, and fluid loss agent SYJ-2 are all provided by Oil Service Company. Table 1 shows the composition of Jiahua G-grade oil well cement.
.png)
1.2 Synthesis of Retarder HFB-2
To prevent the degradation of polymer in high temperature, high pressure, and strong alkaline environments, the improvement of the thermal stability of the main chain of the retarder molecule is crucial. Therefore, carbon carbon double bonds (C=C) with high bond energy and good stability in high temperature and strong alkaline environments can be selected as covalent bond main chains. Secondly, in order to ensure the excellent retarding effect and sustained heat resistance stability of polymer, attention should be paid to the stability and retardancy of side chain functional groups at high temperatures. Therefore, for polymer based retarders, functional groups with hydrophilicity, adsorption, and chelation properties should be selected, such as carboxylic acid groups (- CH2COO -) and sulfonic acid groups (- SO3-).
Accurately weigh 8.0g of acrylamide (AM), 10.0g of 2-acrylamido-2-methylpropanesulfonic acid (AMPS), and 8.0g of dimethyldiallylammonium chloride (DMDAAC) and place them in a three necked flask with mechanical stirring and cooling reflux device. Add an appropriate amount of deionized water at a stirring rate of 100~200r/min to fully dissolve AM and AMPS. Adjust the pH of the reaction system to 6.5-7.0 using a 40.0% NaOH solution by mass fraction. Then, wait for the water bath temperature to rise to the preset temperature of 75~85℃ before adding the initiator ammonium persulfate (APS) solution and maleic anhydride (MAH) solution dropwise. Control the dropwise addition time to 30 minutes. After the drip addition is completed, continue the reaction at the same temperature and stirring rate for 3.0 hours, and then cool to room temperature to obtain HFB-2.
1.3 Characterization of Retarder HFB-2
(1) Infrared Spectrum
Using NICOLET5700 infrared spectrometer for infrared spectroscopy analysis of retarder HFB-2 to characterize its chemical structure. Firstly, take an appropriate amount of spectral pure potassium bromide and retarder HFB-2 after drying treatment, mix them, crush and compress them, and then conduct testing. Among them, the resolution is 4cm-1, the scanning frequency is 32, and the scanning range is 4000~500cm-1. In addition, the retarder HFB-2 needs to be repeatedly cleaned, purified, and dried with anhydrous ethanol and acetone before conducting infrared experiments.
(2) Gel Chromatography
The molecular weight and distribution of the retarder HFB-2 were determined using an IC761 ion chromatograph. Prior to testing, the powdered retarder HFB-2 must be prepared into a polymer solution.
(3) Thermogravimetric Analysis
The thermal stability analysis of retarder HFB-2 was conducted using STA449F3 Jupiter synchronous thermal analyzer, with a testing temperature range of 30-800 ℃, nitrogen atmosphere, and a heating rate of 10℃/min.
(4) X-ray Diffraction
X-ray diffraction analysis was performed on cement powder samples using an X Pert PRO MPD X-ray diffractometer. Targeting Cu with X-ray wavelength λ= 0.1541837nm, working voltage 40kV, current 30mA, scanning angle 2θ=5~70°, cement stone powder samples are taken from the cross-sectional structure of solidified cement slurries with different formulations.
(5) Scanning Electron Microscopy
The microstructure analysis of cement paste was carried out using ZEISS EVO MA15 scanning electron microscope, and the test samples were taken from the cross-sectional structure of solidified cement slurries with different formulations. Before testing, it is necessary to spray gold on the cement stone sample to enhance its conductivity.
1.4 Performance Testing of Retarder HFB-2
The preparation of cement slurry, initial consistency, thickening time, and compressive strength testing are all carried out in accordance with GB 10238-2005 "Oil Well Cement", and the rheological properties are carried out in accordance with SY/T 5504.3-2008 "Evaluation Methods for Oil Well Cement Additives Part 3: Drag Reducing Agents".
2. Results and Discussion
2.1 Synthesis and Analysis of Retarder HFB-2
During the research process, the influencing factors involved in the synthesis of retarder HFB-2, such as monomer ratio, initiator dosage, reaction time, reaction temperature, monomer concentration, and drip time of maleic anhydride solution, were studied using orthogonal experimental design and single factor experimental method, respectively. The optimal synthesis process conditions of retarder HFB-2 were determined (Table 2).
.png)
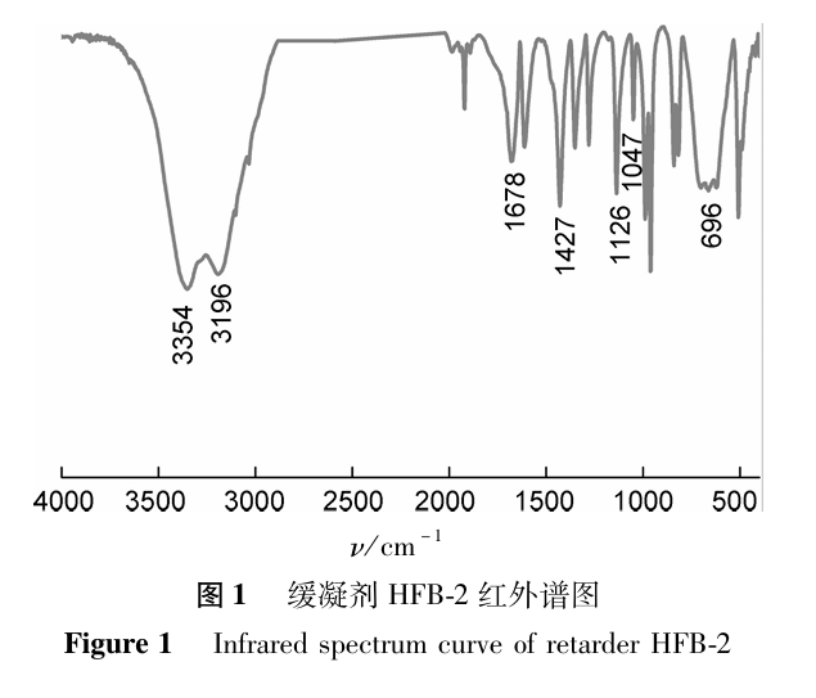
2.2 Infrared Spectroscopic Analysis of Retarder HFB-2
The infrared spectrum of retarder HFB-2 is shown in Figure 1. As shown in Figure 1, the two characteristic absorption peaks at 3354cm-1 and 696cm-1 correspond to the N-H and C=O stretching vibration absorption peaks in the AM structural unit, respectively, indicating that AM participates in the reaction and successfully polymerizes. The characteristic absorption peak at 1126cm-1 has a higher intensity and narrower peak shape, which is consistent with the C-S stretching vibration absorption peak characteristics in the AMPS structural unit. At the same time, combined with the characteristic absorption peak at 1047cm-1 of -SO3H, it indicates that AMPS participates in the reaction and successfully appears in the target product. The characteristic absorption peak at 1678cm-1 corresponds to the C=O stretching vibration absorption peak in the MAH structural unit, indicating that MAH participates in the reaction and successfully polymerizes. In addition, at 3196cm-1, there is a characteristic peak of N-H bond stretching vibration for amide groups, and at 1427cm-1, there is a characteristic peak of N-H bond stretching vibration for quaternary ammonium salts, indicating that the DMDAAC structural unit has successfully participated in the reaction. From the above, it can be seen that acrylamide (AM), 2-acrylamide-2-methylpropanesulfonic acid (AMPS), maleic anhydride (MAH), and dimethyldiallylammonium chloride (DMDAAC) have successfully undergone polymerization reactions under the action of initiators.